In the rapidly growing and highly competitive health supplement industry, conducting a Supplement Validation Study is becoming increasingly essential for companies to establish trust and credibility with consumers. With a multitude of products and brands on the market, consumers are demanding scientific evidence that supports the efficacy and safety of supplements. In this article, we will explore the importance of Supplement Validation Studies, the challenges faced by health supplement companies, and the steps to conduct a successful study.
Introduction: The Health Supplement Industry
The health supplement industry is booming, with over 29,000 dietary supplements available in the U.S. market and approximately 1,000 new products being introduced every year. Despite its growth, the industry faces challenges in gaining consumer trust, primarily due to limited regulation and the presence of inflated claims, supplement brands that tarnish the reputation of legitimate companies. In response, health supplement companies must conduct Supplement Validation Studies to provide scientific evidence of their products’ efficacy and safety, ultimately helping consumers make informed decisions.
Consumer Demand for Scientific Evidence
As consumers become more health-conscious and educated about supplements, they are increasingly demanding scientific evidence to support the efficacy of products. According to a survey, 59% of global consumers want to see scientific evidence supporting supplement efficacy. This increasing demand for evidence-based supplements has led to the need for Supplement Validation Studies, which provide objective data on a product’s effectiveness and safety.
The Role of Supplement Validation Studies
A Supplement Validation Study is a research process in which a health supplement company tests its products’ efficacy, safety, and quality. These studies aim to provide scientific evidence that supports the company’s claims about its products, helping to build trust with consumers and differentiate the brand from competitors.
By conducting a Supplement Validation Study, health supplement companies can:
- Validate the effectiveness of their products
- Ensure product safety and quality
- Build consumer trust and credibility
- Comply with regulatory requirements
- Gain a competitive edge in the market
Challenges in Conducting Supplement Validation Studies
Health supplement companies face several challenges when conducting Supplement Validation Studies, from finding suitable participants and partners to managing costs and navigating regulatory requirements.
Participant Recruitment
One of the primary challenges in conducting a Supplement Validation Study is finding suitable participants who meet the study’s criteria. This can be a time-consuming and expensive process, especially for companies that do not have a large customer base or access to a participant pool.
Cost Management
Another challenge is managing the cost of conducting the study, including participant recruitment, data collection, and analysis. As health supplement companies often operate on tight budgets, finding cost-effective solutions for conducting these studies is crucial.
Finding the Right Partners
Health supplement companies may require partnerships with research institutions, laboratories, or other organizations to conduct their Supplement Validation Study. Finding the right partners with the necessary expertise and resources can be a difficult and time-consuming process.
Regulatory Requirements
Companies must comply with various regulatory requirements when conducting Supplement Validation Studies, such as ensuring proper informed consent and ethical research practices. Navigating these requirements can be complex and may require legal or regulatory expertise.
Steps to Conduct a Successful Supplement Validation Study
To overcome these challenges and conduct a successful Supplement Validation Study, health supplement companies should follow these steps:
- Define the study objectives: Clearly outline the goals of the study, such as validating a specific product claim or demonstrating the safety of a new ingredient.
- Develop a study protocol: Design a research study that addresses the objectives, including selecting the appropriate study design, defining the target population, and determining the sample size.
- Identify and collaborate with partners: Find suitable partners with the necessary expertise and resources to conduct the study, such as research institutions, laboratories, or research organizations.
- Recruit participants: Develop a recruitment strategy to find suitable participants for the study, including using targeted advertising, social media, or partnering with patient organizations.
- Collect and manage data: Implement a data management system to collect, store, and analyze data from the study, ensuring that all data is accurate and reliable.
- Analyze and interpret results: Analyze the study data to determine the effectiveness and safety of the supplement, and interpret the results in the context of the study objectives.
- Communicate results: Share the study findings with stakeholders, including consumers, healthcare professionals, and regulatory authorities, to build trust and credibility.
- Comply with regulatory requirements: Ensure that the study is conducted in compliance with all relevant regulatory requirements, including obtaining informed consent and adhering to ethical research practices.
Leveraging Technology and Partnerships for Supplement Validation Studies
To streamline the process of conducting Supplement Validation Studies, health supplement companies can leverage technology and partnerships to overcome the challenges they face.
Utilizing Digital Platforms
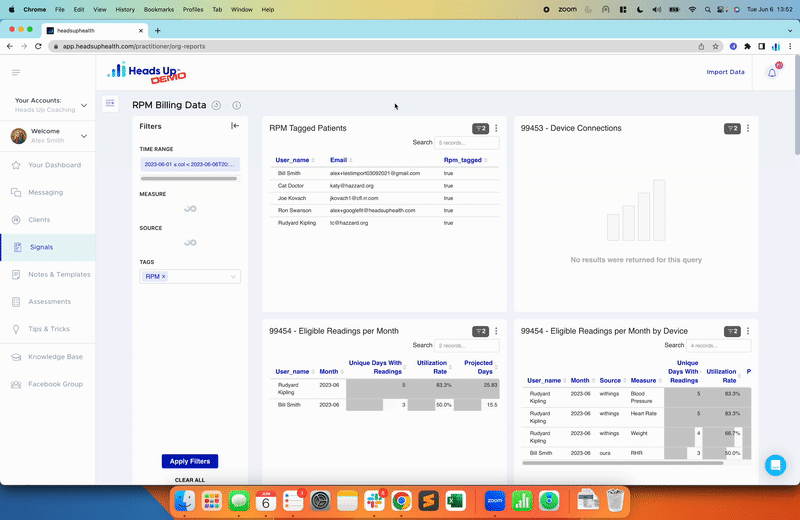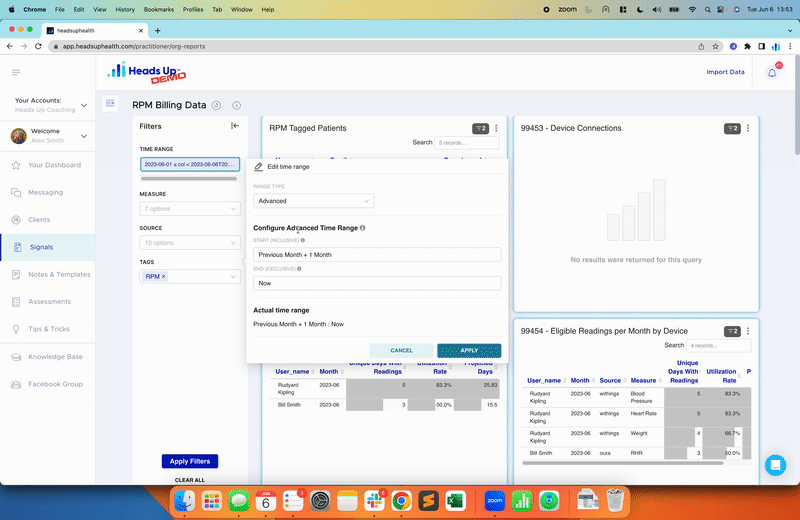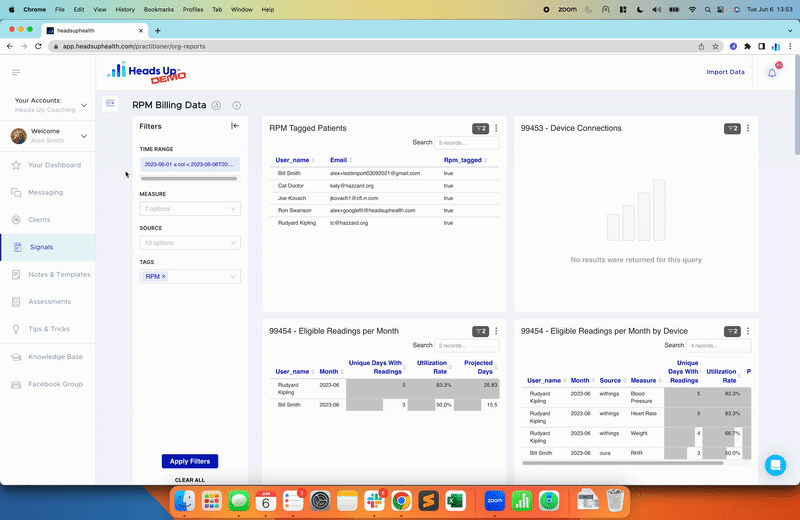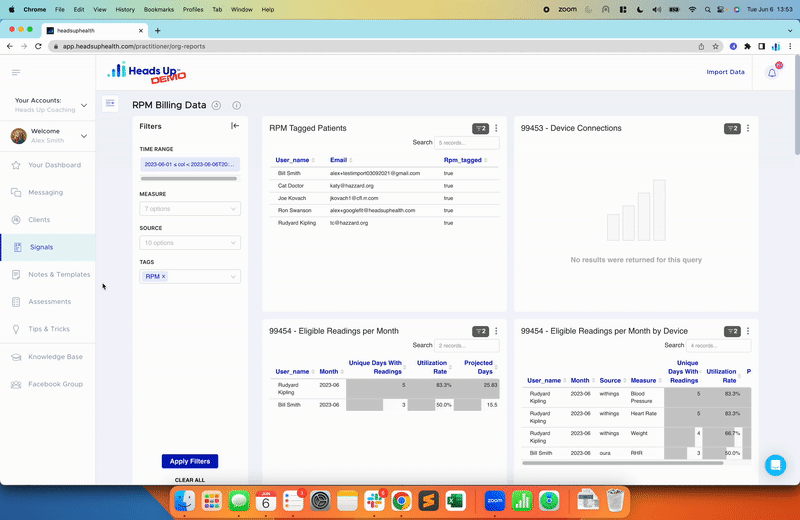
Digital platforms that support data collection and analysis, like Heads Up’s, can help health supplement companies efficiently manage their studies. These platforms can automate data collection from wearable devices, apps, and other sources, reducing the burden on participants and the company itself. Additionally, they can provide real-time data analysis and reporting, enabling companies to monitor trends and make informed decisions quickly.
Partnering with Expert Organizations
Collaborating with expert organizations, such as research organizations, like Heads Up’s research division, can provide health supplement companies with the necessary resources and expertise to conduct successful Supplement Validation Studies. These partnerships can help companies navigate regulatory requirements, recruit participants, and analyze data, ultimately leading to efficient and cost-effective studies.
Conclusion
In the competitive health supplement industry, conducting a Supplement Validation Study is essential for companies to build trust with consumers and differentiate their products from competitors. By overcoming the challenges of participant recruitment, cost management, and finding the right partners, health supplement companies can successfully conduct these studies and provide scientific evidence of their products’ efficacy and safety. By leveraging technology and partnerships, companies can streamline the process and ensure the success of their Supplement Validation Studies.
Click here to learn about HRV+ a recent supplement validation study for ModeByMethod.